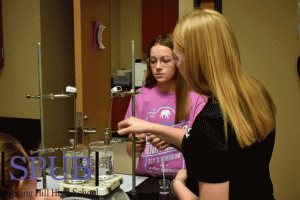AP Chemistry Class Finds Air Density
Ashdon Kice, Photo Manager|December 10, 2019
-
On Dec. 5 during the second hour AP Chemistry class, Wyatt Pollom, 11, takes data for his lab. The lab they are doing is the Molar Mass of Vapor (Photo by H. Smith).

-
In the AP Chemistry class, Travis Leaton and Liz Wilson, 12, prepare their test tube for their lab. The AP Chemistry class is a college level class that replaced the chemistry two class. It goes through advanced chemistry concepts that can earn you college credit (Photo by H. Smith).

-
In the second hour AP Chemistry class on Dec. 5, Jacob Hooker and Evan Letellier, 11, need help setting up their ring stand to hold their test tube. Gissel McDonald, science teacher, helps her students and they go on to complete their molar mass of vapor lab (Photo by H. Smith).

-
During their lab in AP Chemistry on Dec. 5, Morgan D’Albini and Layla Smith, 11, prepare to put their test tube in boiling water. In this lab they are tasked with finding the density of gas through vaporization (Photo by H. Smith).
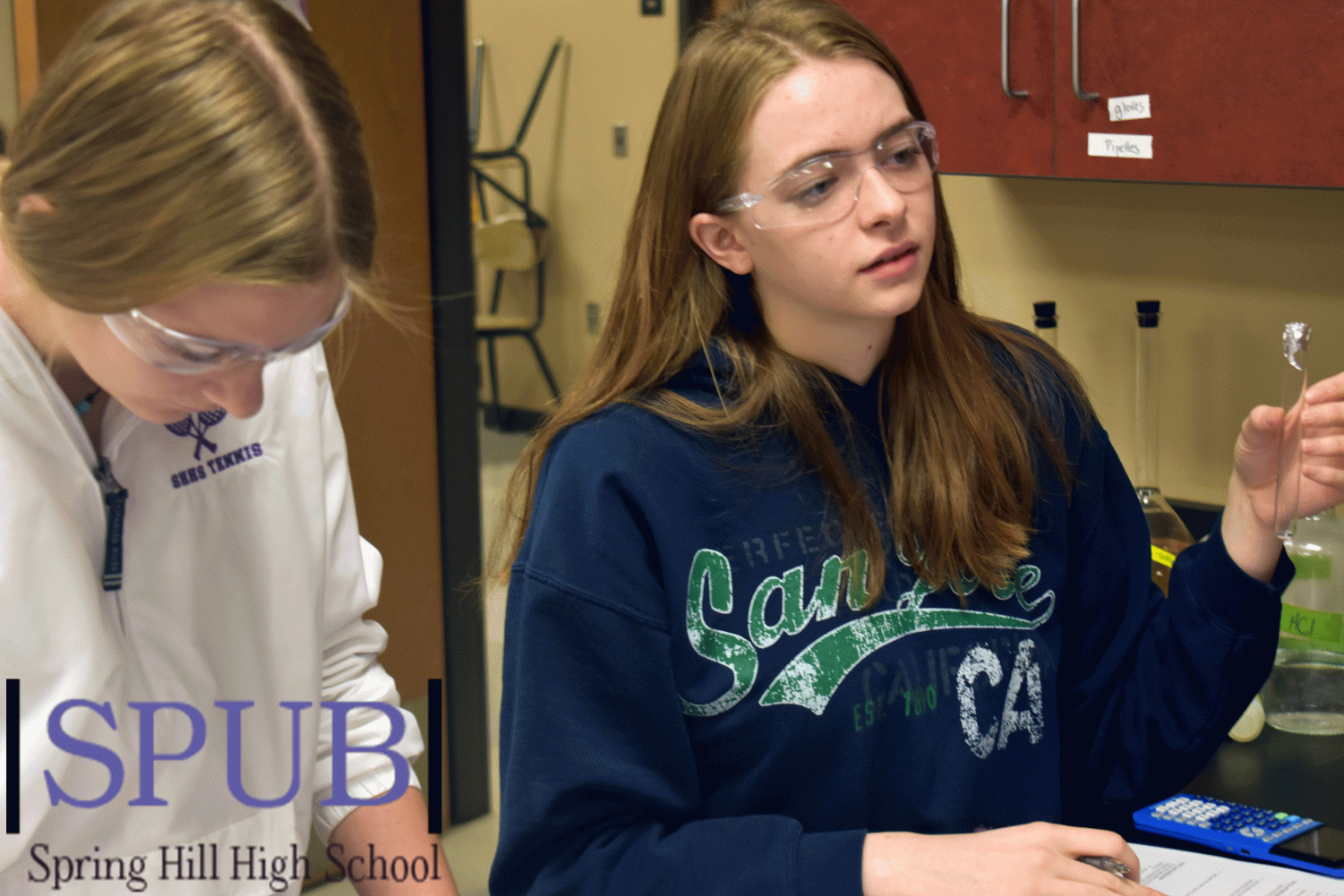
-
For their lab on Dec. 5, AP Chemistry students, John Mitchell and Aidan Torrez, 11, put their test tube in boiling water for a Molar Mass of Vapor lab. In this lab they are trying to find the density of gas through the process of vaporization (Photo by H. Smith).

-
During their Molar Mass of Vapor lab on Dec. 5, Gissel McDonald, science teacher, helps her students, Kyle Hernandez, 12, and Wyatt Pollom, 11, set their test tube after starting off the lab with equipment problems. This lab is part of the AP Chemistry curriculum; they will use the results of this lab to calculate the density of gas (Photo by H. Smith).

-
In their second hour AP Chemistry class on Dec. 5, Kyra Schmuhl and Kristian Nelson, 11, places their test tube into boiling water. They do this to use found numbers of molar mass to find the density of vapor (Photo by H. Smith).
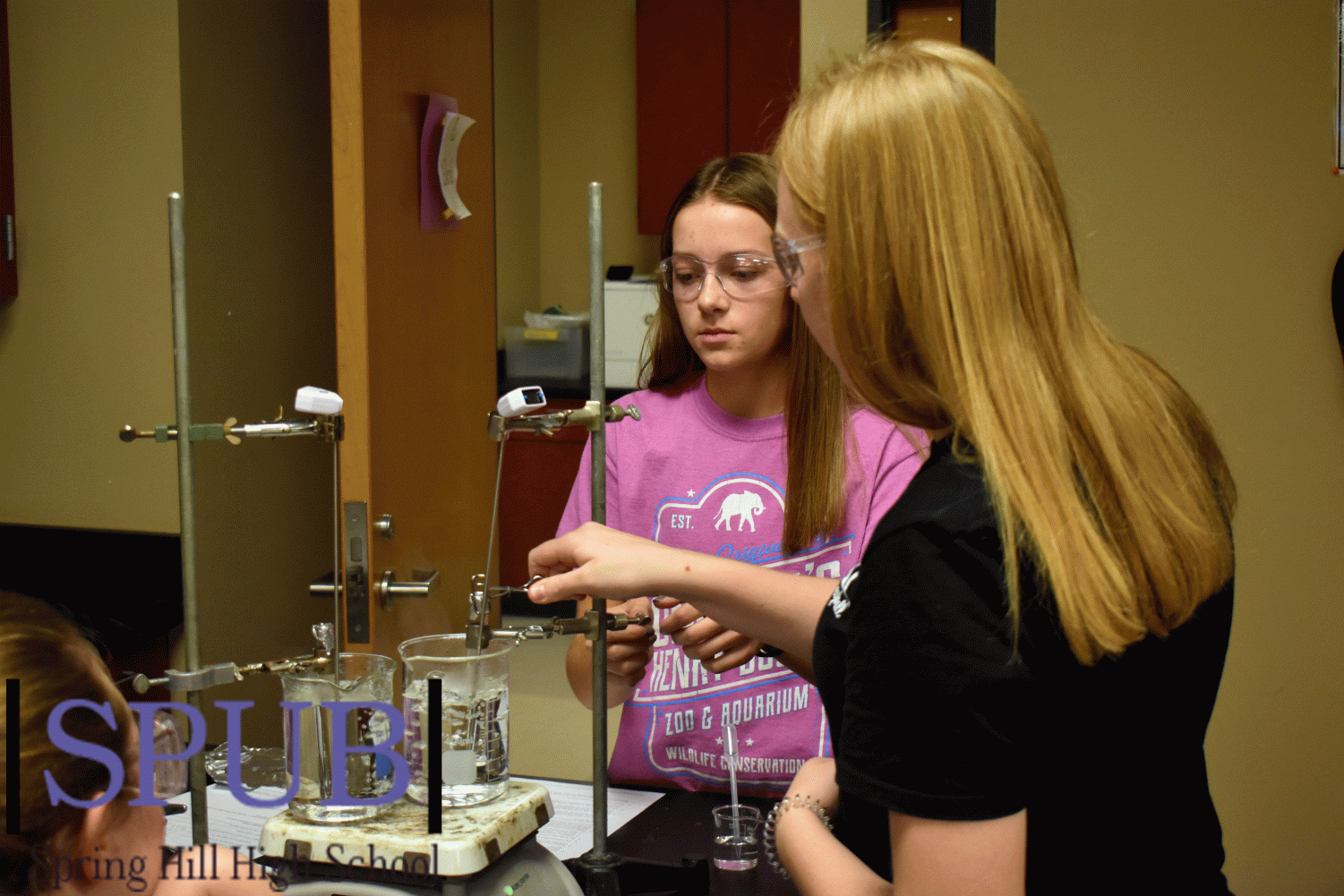
-
While working on a lab in his second hour AP Chemistry class on Dec. 5, Jordan Kinsey, 11, checks to see if the liquid in his test tube has evaporated. Kinsey has his phone ready to record the time it takes (Photo by H. Smith).

-
The AP Chemistry teacher, Gissel McDonald, class Keen Knittle and Jordan Kinsley, 11, set up their test tube for their lab. In this lab they were tasked with determining the density of the gas through evaporation (Photo by H. Smith).

-
Juniors, Keen Knittle and Jordan Kinsley, get ready for their second trial of their Molar Mass of Vapor lab. After completing their first trial they had to repeat it to compare their results (Photo by H. Smith).


Hi, my name is Ashdon Kice, this is my second year as Stampede News' Photo Manager. My job includes creating and posting galleries, along with writing...